Publication details
The paper is published in the Journal of Abdominal Radiology: click here
Abstract
Purpose Liver biopsy was considered the gold standard for diagnosing liver fibrosis; however, with advancements in medical technology and increasing awareness of potential complications, the reliance on liver biopsy has diminished. Ultrasound is gaining popularity due to its wider availability and cost-effectiveness. This study examined the machine learning / deep learning (ML/DL) models for non-invasive liver fibrosis classification from ultrasound.
Methods Following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) protocol, we searched five academic databases using the query. We defined population, intervention, comparison, outcomes, and study design (PICOS) framework for the inclusion. Furthermore, Joana Briggs Institute (JBI) checklist for analytical cross-sectional studies is used for quality assessment.
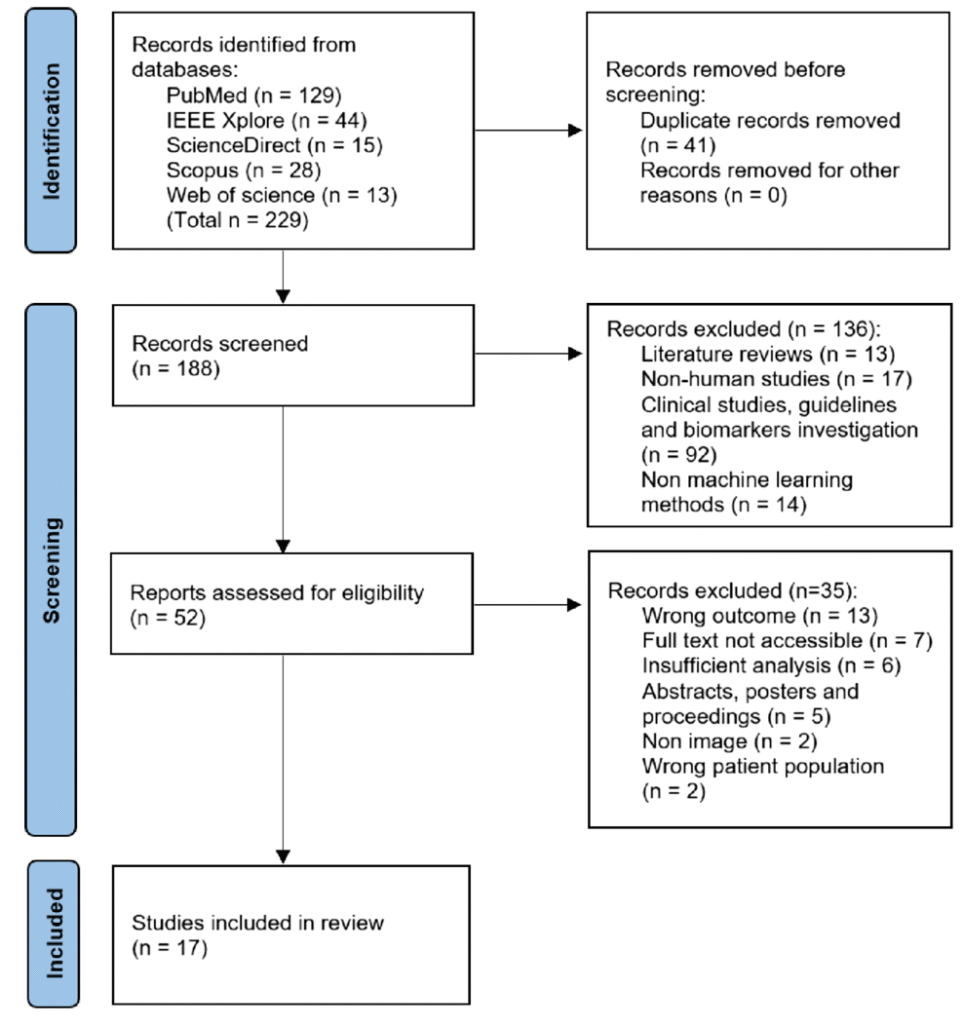
Results Among the 188 screened studies, 17 studies are selected. The methods are categorized as off-the-shelf (OTS), attention, generative, and ensemble classifiers. Most studies used OTS classifiers that combined pre-trained ML/DL methods with radiomics features to determine fibrosis staging. Although machine learning shows potential for fibrosis classification, there are limited external comparisons of interventions and prospective clinical trials, which limits their applicability.
Conclusion With the recent success of ML/DL toward biomedical image analysis, automated solutions using ultrasound are developed for predicting liver diseases. However, their applicability is bounded by the limited and imbalanced retrospective studies having high heterogeneity. This challenge could be addressed by generating a standard protocol for study design by selecting appropriate population, interventions, outcomes, and comparison.
PRISMA flowchart for the search, filter, and inclusion of the studies in the systematic review
Findings
We established that the research community has increased efforts at developing non-invasive diagnosis practices with automated ML/DL solutions using ultrasound, particularly for predicting liver diseases. Beyond research, these advances can profoundly benefit clinical practice by offering safer and more patient-friendly diagnostic alternatives, enabling earlier interventions and potentially improving patient outcomes. However, the transition from theoretical promise to clinical reality remains elusive with challenges. Notably, the lack of prospective clinical trials and comprehensive external validation in the studies underline a crucial gap. To ensure these technologies realize their full potential and guarantee genuine clinical utility, it is imperative to bridge this gap, perhaps by introducing standard protocols for study design and robust validation methodologies.
